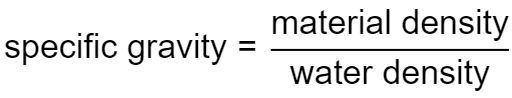Specific Gravity Calculator
Welcome to the specific gravity calculator, a tool with which you'll be able to perform density to specific gravity calculations.
In the following sections, we present the specific gravity definition and formulas, and you'll learn how relative density can help determine if a material floats or sinks.
Specific gravity definition
By definition, the specific gravity of a material is the ratio of its density to the density of some reference material:
SG = ρmaterial/ρreference
where:
- ρmaterial — Density of the material whose specific gravity will be calculated, in kg/m3;
- ρreference — density of the reference material, in kg/m3; and
- SG — specific gravity or relative density, without units;
For liquids and solids, the reference density is usually the density of water at 4 °C (39.2 °F), which is 1000 kg/m3, the maximum possible value for it. For gases, the reference density is typically air density at room temperature (20 °C or 68 °F), which equals 1.204 kg/m3.
🙋 As you can see, specific gravity has no relation to gravity. Therefore, a more appropriate name is relative density.
Specific gravity formula used by this calculator
From the previous definition, taking the reference material as water at 4°C, we can state the specific gravity formula:
-
SG = ρmaterial/ρwater@4°C, or
-
SG = ρmaterial/(1000 kg/m3)
🙋 Although gases are common substances, when we say "specific gravity," we usually refer to a liquid. Therefore, this calculator always uses water density as the reference and assumes you need to calculate the specific gravity of a solid or liquid material.
For example, to calculate the specific gravity of iron (whose density is 7870 kg/m3):
SGiron = (7870 kg/m3)/(1000 kg/m3) = 7.87
From the specific gravity formula, we can note something:
- If SG > 1, the material density is higher than water density, and the material sinks into the water.
- If SG < 1, the material density is lower than water density, and the material floats into the water.
- If SG = 1 (relative density equals one), the material density is the same as the water density.
Specific gravity and density of many common materials
In the following table, you can look at the density of many common materials, as well as their specific gravity, calculated by taking water density (1000 kg/m3) as the reference density:
Material | Density (kg/m3) | Specific gravity |
|---|---|---|
Balsa wood | 2000 | 0.2 |
Oak wood | 750 | 0.75 |
Ethanol | 780 | 0.78 |
Olive oil | 910 | 0.91 |
Ice | 910 | 0.91 |
Vodka | 949.8 | 0.9498 |
Water | 1000 | 1 |
Blood | 1060 | 1.060 |
Table salt | 2170 | 2.17 |
Aluminum | 2700 | 2.7 |
Cement | 3150 | 3.15 |
Iron | 7870 | 7.87 |
Copper | 8960 | 8.96 |
Lead | 11350 | 11.35 |
Mercury | 13560 | 13.56 |
Depleted uranium | 19100 | 19.1 |