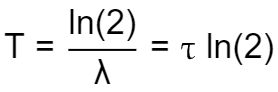Half-Life Calculator
Whether you are considering radioactive atoms or a population of bacteria, the underlying mathematics is the same: our half-life calculator will teach you how to compute the most important quantity of the decay process, the half-life.
Here you will learn:
- What is the half-life in exponential decays;
- How to calculate the exponential decay using the half-life;
- How to calculate the half-life;
- Examples of half-life in physics and biology.
Decay processes
A decay process is any process that sees a decrease in the measured quantity. Decay processes are usually divided into two categories:
- Exponential decays; and
- Non-exponential decays, where we can identify subtypes like:
- Inverse square decay; or
- Zipf's law decay
What is the half-life?
In any decay process, we can pinpoint a moment in which the quantity is half the original amount. We call the time elapsed from the starting moment to this point the half-life of the quantity.
We can define the half-life also in terms of probability. This approach best suits discrete quantities or unitary ones. In this case, the half-life is the time after which there is a chance of decay.
In physics, the half-life usually describes stochastic processes as radioactive decay, where an unstable atom emits or absorbs a particle to change species. This process is entirely random, and an atom can go eons without decaying. On the contrary, in biology, a bacteria in a decaying population will die after a given time: no immortal bacteria out there.
How do I calculate the half-life?
Let's learn how to calculate the half-life of an exponential decay (a mathematical model based on the exponential growth).
We define first the law of exponential decay:
Where:
- is the quantity at the time ;
- is the quantity at the initial reference time;
- is the elapsed time; and
- is the average lifetime of each component of the measured quantity.
🔎 , the average lifetime, is often expressed as its inverse, the decay constant : .
To find the half-life we can rearrange this expression, knowing that, when the half-life time is reached:
We can define the half-life time as:
Which can be easily rewritten to isolate using the natural logarithm:
Using this relation, we can write the half-life equation using the factor characteristic for the halving of the quantity:
Our half-life calculator works in both directions: you can calculate the half-life of a decay process if you know the initial and final quantities, and the elapsed time, or you can calculate the final (or initial) quantities if you know the half-life.
Examples of how to calculate the half-life
We will calculate the half-life in two situations: radioactive decay and the decline of a bacterial population.
Take a radioactive atom, let's say promethium (were you expecting uranium?). Promethium is the lightest natural radioactive element. Its most common (and stable) isotope, , has half-life . Scientists estimate that on Earth, there are about of promethium at a given time. Assuming that no more atoms of this element would form, how much promethium would we have after ? Input the known data in the half-life equation:
Let's try the other way around. What's the half-life of a bacteria population that starts with individuals, and after a day ends with individuals. Input the data in our half-time calculator:
Now, if you knew the energy released after , you could calculate the power to estimate how many watts such a decay generates. Then, might be the next step to express power in more adequate units. That would be very interesting to check!